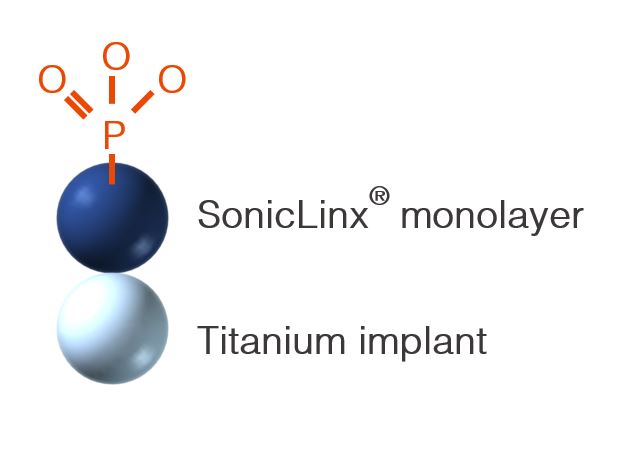
SonicLinx® - The Natural Implant Interface™
Key Points
- Highly hydrophilic implant surface:
- Reduces protein denaturation and inflammatory reactions to the implant
- Implant integration without fibrous capsule formation
- Bio-mimicking surface:
- Early bone growth on the implant surface will result in faster osseointegration and can allow for earlier loading
- Increased neo-vascularization promotes healthier bone
- Reduced risk of implant loosening even with poor bone quality
Technical Facts
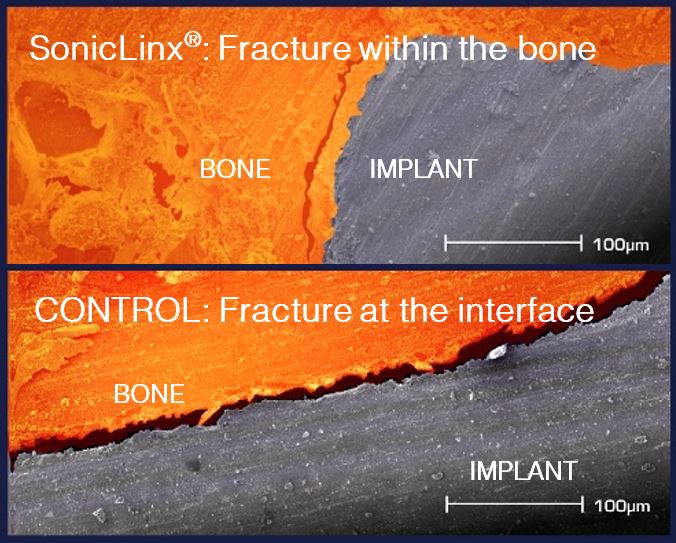
Clinical Facts
Individualized Solutions by VetWelding
Bring personalized medicine to the realm of veterinary orthopedics. Make surgery perfect with
customized planning, patient-specific implants and individualized surgical guides.